Project Management
Project Management Structure
The aim of the ALPHA-MAN proposal is to develop and perform clinical trials using rhLAMAN as a therapeutic drug for the inherited disease alpha-Mannosidosis for which limited treatment is currently available. In addition, the ALPHA-MAN project will help to understand the impact of chronic ERT treatment on CNS neuropathology in alpha-Mannosidosis mice. This preclinical ERT study will also help to realize the mechanism how rhLAMAN crosses the Blood Brain Barrier and reaches the brain. To achieve this goal the ALPHA-MAN consortium has been developed and will bring together 12 European partners including scientists, clinicians and industry. Most of the partnes involved in ALPHA-MAN have already contributed to the previous European consortia EURAMAN and HUE-MAN and therefore are experienced in EU networks and regulations. Every partner possesses a special expertise and knowledge within its research field and all partners will work closely together. Teamwork within the partners of the different WP form the basis for the successful outcome of the ALPHA-MAN project. To achieve the best results intensive feedback between the participants on a research level, is essential. In addition, discussions on technological development, innovation and exploitation of the results is also required. To accomplish this successful collaboration within the WP and to guarantee maximal outcome of the ALPHA-MAN project, smooth management of the consortium is required. The management of the consortium is grouped in the following committees:
- Work Package Leader (WP leader)
- The Clinical Trial and Industrial & Innovation Management Board (CCB and IIMB)
- The Project Management Team (PMT)
- Project Coordination Committee (PCC)
- The Co-ordinator
The following graph represents the interaction and activities within the defined management committees.
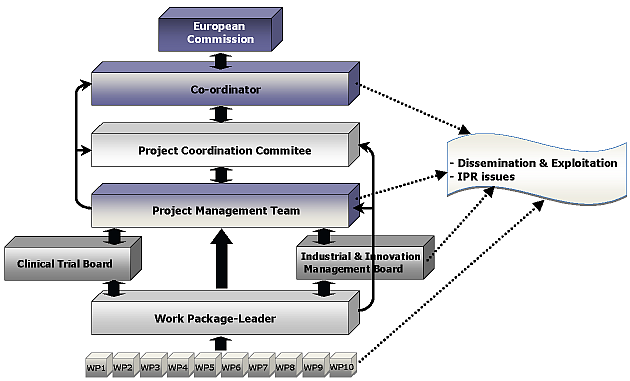
Work Package Leader
Each workpackage will have a nominated Work Package Leader being responsible to the consortium, represented by the Project Coordination Committee, for all aspects that are specific to the single work package. WP Leaders will be responsible for day-to-day management of their respective WPs. On the scientific level the tasks of WP leader include: i) propagation of WP results, ii) ensuring the scientific quality of the work within their WP, iii) time schedule management, iv) assuring that the respective deliverables and milestones of the WP are achieved, and v) discussion and solving problems with the scientist and participants of the WP.
On the administrative level WP leaders are responsible for: 1) communication with the PMT or Co-ordinator about the budget allocated to the partners involved in the WP, 2) ensuring the flow of communication inside and outside their WP and 3) on time delivery of the progress and budget reports to the Co-ordinator.
The Clinical Trial and Industrial & Innovation Management Board
Within ALPHA-MAN, industrial and clinical activities are a main part of the project. Due to the clinical trials performed within ALPHA-MAN a great amount of data will be generated that have to be evaluated fast and efficiently in order to guarantee the smooth process of the trials. Many partners will be involved in the clinical trials and due to their complexity, a special response on the project management level is required. Therefore we defined the i) Clinical Trial and ii) Industrial & Innovation Management Board. The Clinical Trial Board will be represented by all clinical partners involved in the clinical trials. Head of this consortium will be Dr. Jens Fogh (Partner 2). The Industrial & Innovation Management Board will consist of all industrial partners and head of this Board will be Pia Ringholm (Partner 2). The duties and activities of these two consortia are outlined below.
Clinical Trial Board
Alpha-Mannosidosis is a rare disease and due to the low number of patients worldwide, the performance of clinical trials will be a challenge that requires maximal organization and management. In total approximately 30 patients (aged 6 -20 years) are required to perform the clinical trials and these patients must have the physical ability and mental capacity to perform the endurance (6-minute walk test) and lung function test as these parameters will be used to monitor the efficacy of the ERT treatment. We will establish five clinical sites in Europe (Denmark, Germany, France, Poland and United Kingdom) where the clinical trials will take place. This implies that the patients that have been chosen by the Clinical Trial Board to participate in this study have to travel to the nearest clinical site where they will be treated. Patients will be injected every week and they will have to be assissted by their parents or another person in charge. The organization of the travelling of patients and their parents to the clinical sites will be a significant part of clinical trial management. The Clinical Trial Board will help all partners involved in this study to organize their individual clinical trial and will discuss problems that arise during the study and will help to find solutions. The Clinical Trial Board will be also responsible for making the decision as to which patients are most suitable for the trial as patient selection may prove crucial to the outcome of the study. The clinical trial board will also manage ethical issues (Section 4) that were raised within the project. The Head of the Clinical Trial Board will be Dr. Jens Fogh (Partner 2) who has long-standing experience in the development and implementation of clinical trials for rare diseases. The first Clinical Trial Board meeting will be held 6 months before the project commences in order to identify suitable patients for the study and to ensure that when the project starts all clinical sites are ready and have enough patients to be tested. Further Board meetings will be held after the phase I and II trials have been completed, to discuss and evaluate the data collected during the initial trials and to decide the best protocol for the phase III.
Industrial & Innovation Management Board
The Industrial & Innovation Management Board will assure that the analysis of data and the protection and implementation of the results strictly follows the Consortium Agreement. Regular Meetings of the board will help all industrial partners to better organize and combine their activities towards a rapid and efficient analysis of the clinical data. Head of the Industrial & Innovation Management Board will be Pia Ringholm (Partner 2) who is experienced in clinical development, production, quality assurance and international regulatory affairs. Pia Ringholm will be responsible for the smooth action of all industrial activities and that all data related to clinical trials are analysed on time. She will also be responsible for the patenting process of any results obtained by clinical data analysis.
The Project Coordination Committee (PCC)
There will be a Project Coordination Committee that represents all partners. Therefore each Partner will nominate one representative to the Project Coordination Committee with due authorisation to discuss, negotiate and decide on actions proposed by the Co-ordinator and the Project Management team. Usuallythe representative of the partner within the Project Coordination Committee would be the WP leader themselves. The Project Coordination Committee acts as the decision making body for the ALPHA-MAN Project and will discuss and accept recommendations made by the representative within the frame of its responsibilities. The decisions of the Project Coordination Committee will be binding to all Parties in Project-related matters, which will be clearly constituted within the Consortium. Meetings of the Project Coordination Committee will be held at the invitation of the Co-ordinator at least once a year. In its yearly meeting the Project Coordination Committee shall consider, receive and approve the accounts for the past (financial) year, approve the budget and Implementation Plan for the next (financial) year and decide on changes in work shares as well as acceptance of new parties or withdrawals or exclusion of parties. The Project Coordination Committee can set up Panels to advise and support the Co-ordinator in the proper management and co-ordination of the Project. These Panels will have an advisory role only.
The Project Management Team (PMT)
In contrast to the previous networks EURAMAN and HUE-MAN, the ALPHA-MAN is more diverse, since it is divided into three different bodies including a clinical (clinical trials), scientific (preclinical mouse studies) and industrial part (clinical trial data mangement and analyses). Each body contributes to the project to the same extent. In addition, ALPHA-MAN contains more partners all of Europe, than the previous networks and the organization and management of each WP requires long-standing experience and knowledge.
Due to its complexity and larger scale, ALPHA-MAN requires a robust organization to assure the best performance and outcome of this project. Therefore we defined a PMT consisting of partner 1 and 2 that will help the Co-ordinator to manage the scientific (Partner 1), clinical (Partner 2) and industrial (Partner 2) activities and the consortium in general. The management team will be in a continous contact via email, phone conferences and internal meetings to exchange information, discuss internal issues, and solve problems.
The members of the PMT will be Prof. Dr. Paul Saftig (Partner 1), Dr. Jens Fogh (Partner 2) and Dr. Judith Blanz (Partner 1).
Scientific coordination
Partner 1 is from an academic institution (CAU, Germany) and has a long-standing experience and knowledge about preclinical ERT studies in alpha-Mannosidosis mice and lysosomal storage disorders in general. Partner 1 has generated and bred all alpha-Mannosidosis mouse models available and perfomed all previous ERT mouse studies during the EURAMAN and HUE-MAN networks. Within ALPHA-MAN, partner 1 will play the central role for chronic ERT studies in the immune-tolerant alpha-Mannosidosis mice and will responsible for the overall coordination of the scientific part including preclinicial mouse studies and the subsequent analyses including partner 4 and 9.
Industrial and clinical coordination
The industrial partner 2 that will be responsible for the coordination of the clincial trials and industrial activities has already been part of the previous networks EURAMAN and HUE-MAN. Partner 2 has produced the recombinant enzyme rhLAMAN as a therapeutic agent that will now be used for Clinical Trials in man and mice. Partner 2 is experienced in production of therapeutic drugs especially lysosomal enzymes as well as in organization, implementation and data management of natural history studies and clinical trials. In the past, partner 2 has has already successfully set up natural history studies and clinical trials for other rare diseases such as Metachromatic leukodystrophy (MLD) and Acute Intermittent Porphyria (AIP).
The Co-ordinator
